About Course
Course Duration: 2 Days – 8 Hours/day
This two-day seminar serves as an overview, and upgrade, of the new ISO/IEC 17025:2017 International Standard, which is the recognized international requirements for the competence of testing and calibration laboratories. The technical requirements for laboratories are enhanced by the use of a management system that incorporates the requirements of ISO 9001:2015.
The acceptance of testing and calibration results between countries and industries should be facilitated if laboratories want to comply with this International Standard. The use of this standard will facilitate cooperation between laboratories and other bodies and assist in the exchange of information and experience, standards and procedures.
Course Outline
Day One
- Introduction and Welcome
- Introduction to ISO/IEC 17025:2017
- What’s Changed and Improved
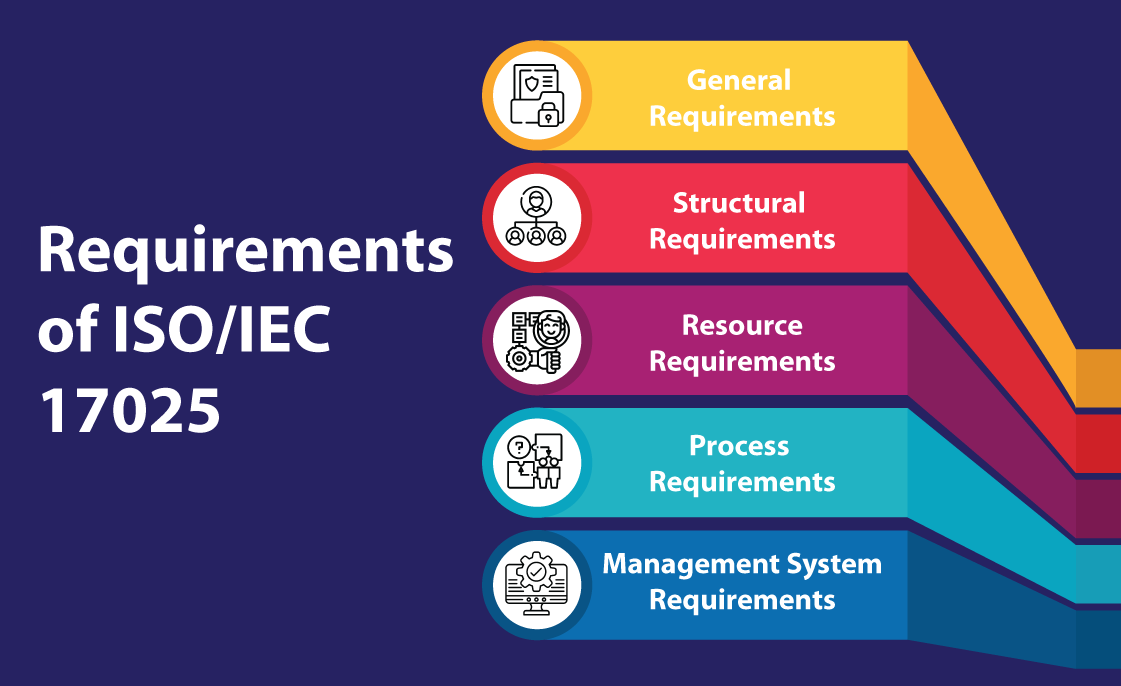
- Overview of ISO/IEC 17025:2017 Requirements
- Group Exercises: Audit Scenarios (cont’d)
Day Two
- Overview of ISO/IEC 17025:2017 Requirements (cont’d)
- Group Exercises: Audit Scenarios (cont’d)

