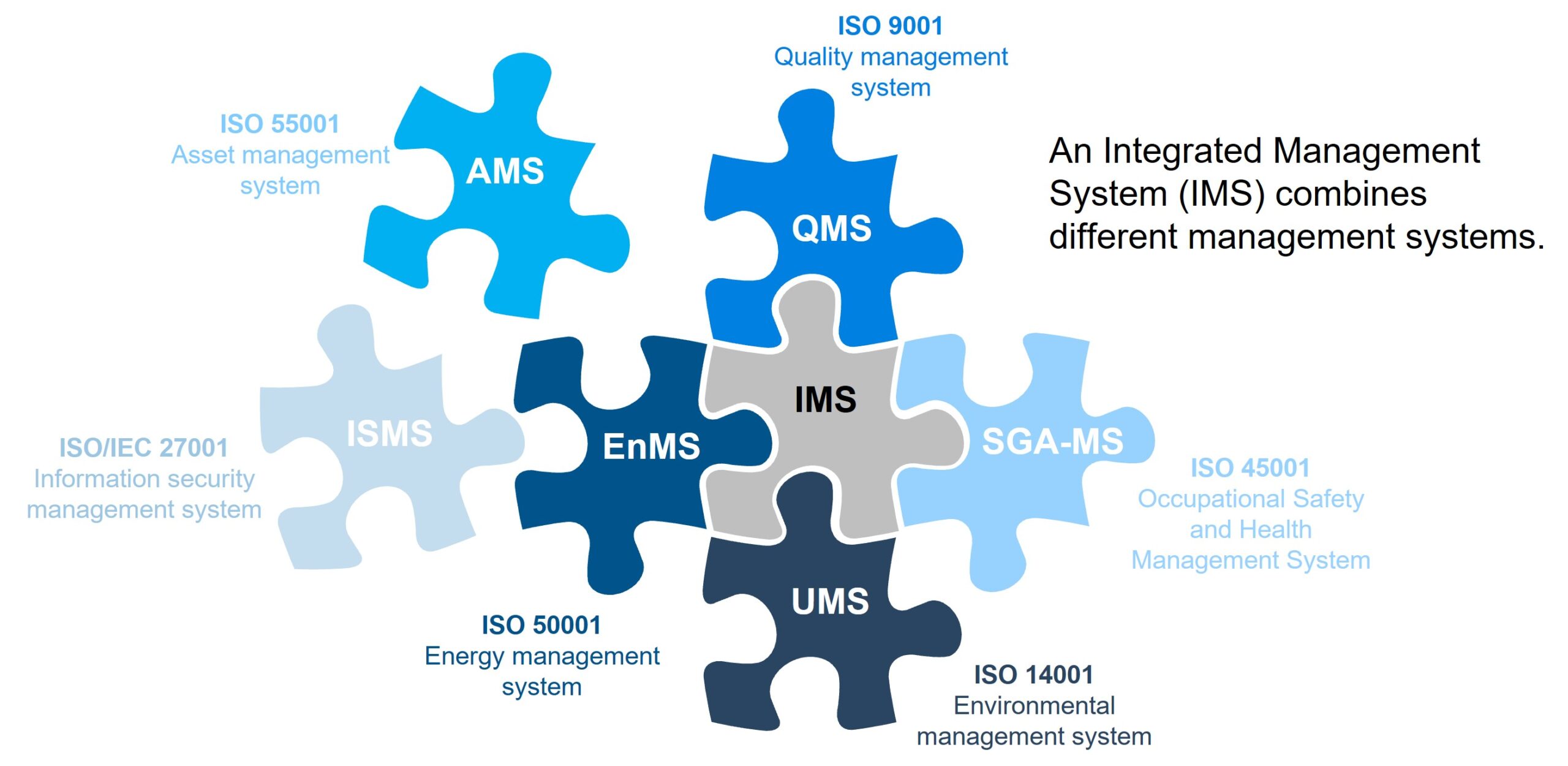
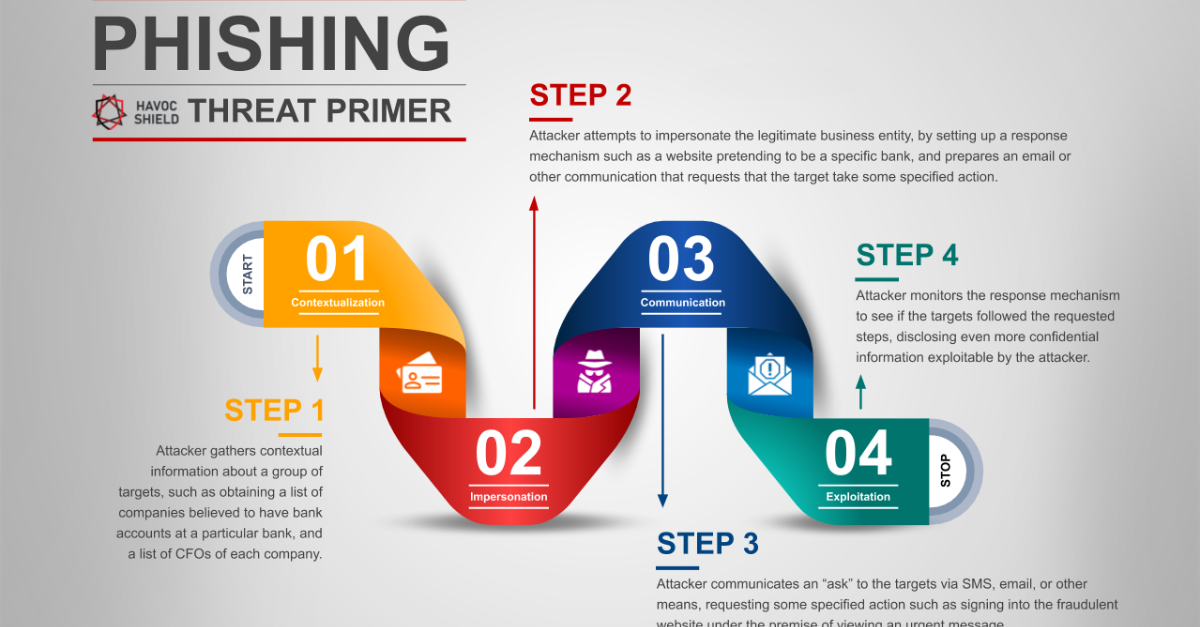
What Is SOC 2? SOC 2 (System and Organization Controls 2) is a cybersecurity compliance framework developed by the AICPA (American Institute of Certified Public Accountants). It’s designed for service providers that store, process, or transmit client data, especially in...