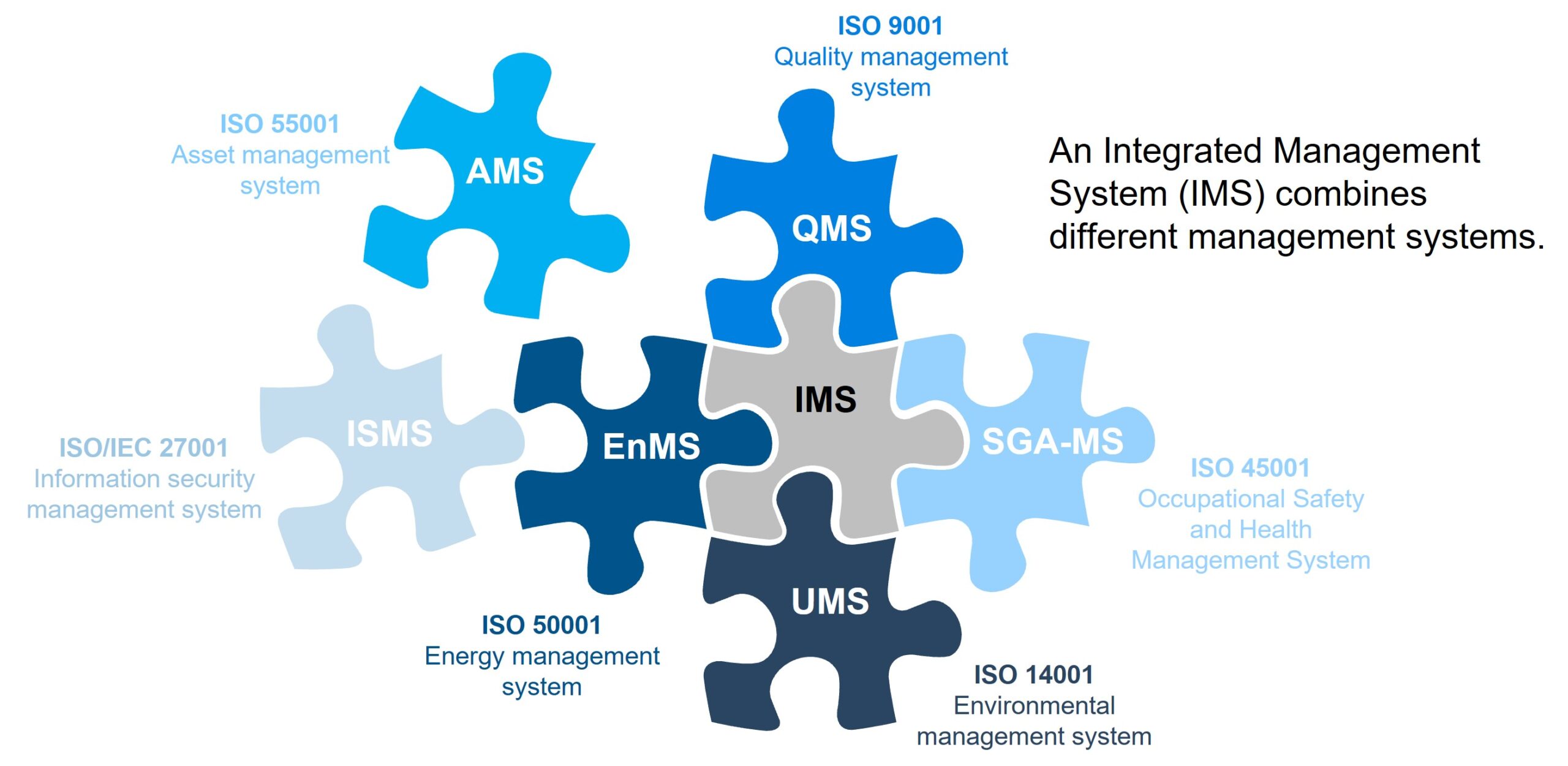
In the rapidly advancing world of medical technology, software plays a critical role in ensuring the safety and performance of medical devices. ISO 62304 is the internationally recognized standard that governs the software life cycle processes for medical device software. Whether you’re a developer, QA engineer, or regulatory professional, understanding ISO 62304 is essential to building safe, reliable, and compliant software.
🔍 What is ISO 62304?
ISO 62304 outlines the framework for developing and maintaining medical device software, covering everything from initial concept and design to maintenance and problem resolution. It ensures that medical software is developed under a controlled and risk-managed environment, reducing potential harm to patients and users.
📘 Why ISO 62304 Training Guide Matters
A structured ISO 62304 training guide helps organizations:
- Understand software classification (Class A, B, C based on risk)
- Implement compliant development processes
- Improve traceability, documentation, and validation
- Meet the requirements of regulators like FDA and MDR
- Build safer software and speed up market approval
🛠 Who Needs It?
- Medical Device Manufacturers
- Software Developers in Healthcare
- Quality Assurance Teams
- Regulatory Affairs Professionals
🌍 ISO 62304 in Pakistan
In Pakistan, growing innovation in medical IT makes awareness of ISO 62304 increasingly important. By following an ISO 62304 training guide, local companies can align with global best practices, enhance product quality, and open new export opportunities.